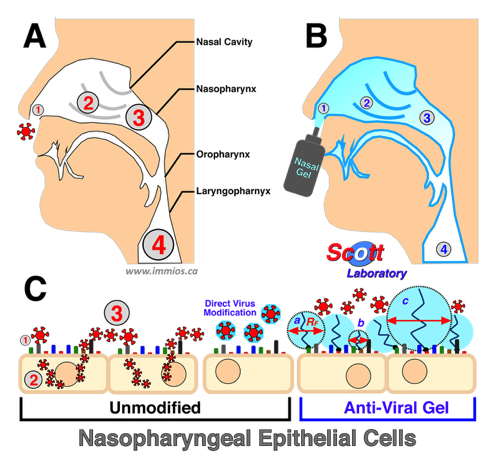
Antiviral effects of nasopharyngeal 'immunocamouflage' by activated polymer gel on disease pathogenesis. Panel A: Normal viral pathogenesis. Panel B: Effect of mPEG-Nasal Gel on viral pathogeniesis. The relative efficacy of the antiviral barrier is denoted by the intensity of the blue shading. In Panels A and B, the size of the number reflects the relative viral number and disease progression: (1) Initial Inoculum; (2) invasion of adjacent cells; (3) production of progeny virus; and (4) disease progression to lower respiratory tract. Panel C: The antiviral effects of grafted polymer are shown at the epithelial cell membrane- environment interface. The efficacy of the grafted polymer is shown by the zone of protection induced by the small (b), medium (a) and large (c) polymers. RF defines the radius of gyration (spatial filling) of the grafted polymer.
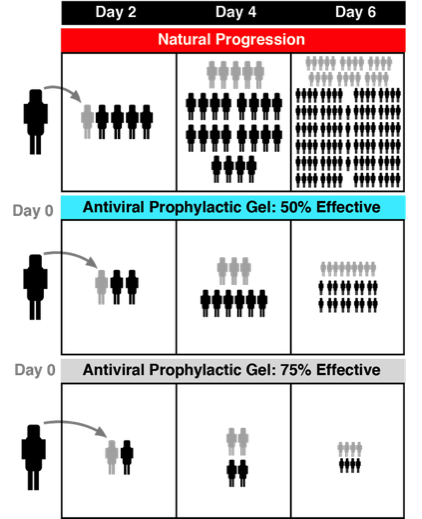
Shown is the natural progression of a hypothetical respiratory disease. Per this model, 1 infected person transmits disease to 4 healthy individuals every 2 days. The previously infected individuals are denoted in grey.
Disease transmission can be dramatically reduced even if the prophylactic nasal gel is not 100% effective at the level of the individual.
Diagrammed is the effect that a 50% or 75% effective nasal gel would have on an at risk population over 6 days. This model assumes that an individual does not die and remains contagious for a minimum of 4 days.
Broad Spectrum Antiviral Gel
Biomitigation Strategy
General Concept of The Intranasal Application of the Anti-Viral Gel
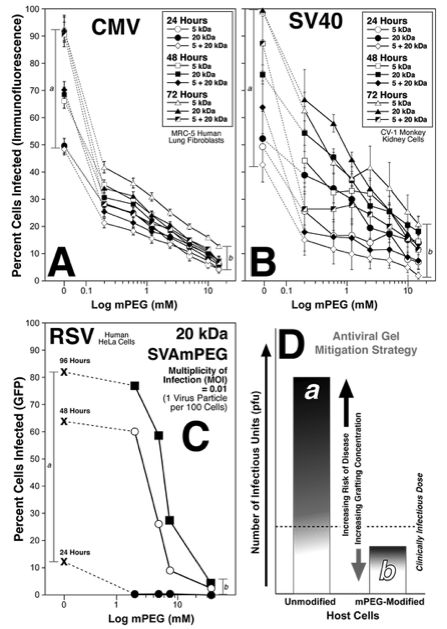
Covalently grafted mPEG exhibits broad spectrum anti-viral efficacy as shown by Percent Infected Cells.
As shown, grafted polymers exhibit a dose dependent decrease in viral invasion and orogeny proliferation of both enveloped (CMV, RSV) and non-enveloped (SV40) viruses. Shown are the antiviral effects of 5, 20, and 5+20 (1:1) kDa polymers on the specific viruses over several days. As noted for each virus, viral infection (and progression via progeny production) of control target cells (a) was dramatically reduced by even low levels of polymer grafting. At the highest grafting concentrations (15 mM; b) significantly reduced viral load was noted for all viruses. Thus, the covalently grafted polymer results in a significant mitigation of viral infection/disease risk and progression as denoted in the lower right panel. Importantly, soluble mPEG (no chemical linker) had no effect on viral invasion or proliferation. N=minimum of 3 independent experiments.
Delivery Device Prototype
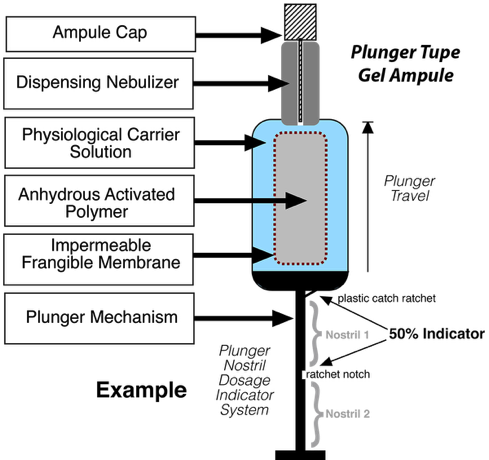
Plunger Delivery Device. The half-life of the activated polymer is very short (e.g., 15-20 minutes) once hydrated posing some delivery issues. To account for this, delivery devices have been designed that allows for the production and stable storage of the nasal gel. As denoted above, the powdered activated mPEG is contained within a frangible membrane within the carrier solution of appropriate pH. When crushed and messaged by finger manipulation, the mPEG becomes dissolved in the solute. The ampule cap is removed and the plunger nozzle placed within the nostril where the gel/spay expelled from the ample by pushing the plunger. The gel/spray passes through a dispensing nebulizer for better coverage of the nasal cavity. This device has a ratchet stop to indicate the appropriate volume for a single nostril. The next depression of the plunger is used for the second nostril. Once dispensed into the nostrils, the gel, due to its viscosity, stays in place. Gentle massing of the nose can be done to more evenly distribute the gel. Due to the rapidity of the covalent bonding, 2-3 minutes after application, the user can blow their nose into a tissue to remove the remainder of the gel.
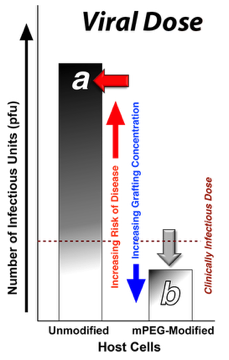
Every virus has a 'minimal' infective dose; i.e., the number of virus particles necessary to induce disease. The grafted polymer barrier reduces the number of viral particles that are able to actually infect cells. As a result, the risk of receiving a high enough viral dose to cause disease is vastly reduced.
Proof of Concept
Kyluik, D.L., Sutton, T.C., Le, Y. and Scott, M.D. Chapter 7: Polymer-Mediated Broad Spectrum Antiviral Prophylaxis: Utility in High Risk Environments. In: Progress in Molecular and Environmental Bioengineering- From Analysis and Modeling to Technology Applications (Editor: Carpi, A.) Intech, ISBN 978-953-307-268-5. pp. 167-190 (2011). DOI: https://doi.org/10.5772/20920
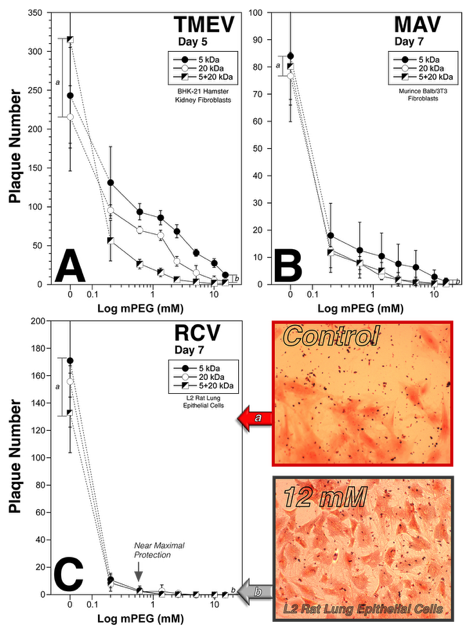
Covalently grafted mPEG exhibits broad spectrum anti-viral efficacy as shown by Plaque Assays.
As shown, grafted polymers exhibit a dose dependent decrease in viral invasion and orogeny proliferation of both enveloped (RCV) and non-enveloped (TMEV, MAV) viruses. Shown are the antiviral effects of 5, 20, and 5+20 (1:1) kDa polymers on the specific viruses over several days. As noted for each virus, viral infection (and progression via progeny production) of control target cells (a) was dramatically reduced by even low levels of polymer grafting. At the highest grafting concentrations (15 mM; b) significantly reduced viral load was noted for all viruses. Thus, the covalently grafted polymer results in a significant mitigation of viral infection/disease risk and progression. Moreover, while control infections resulted in significant cell death (plaques) the grafted cells remained healthy (photomicrographs; lower right panel). Importantly, soluble mPEG (no chemical linker) had no effect on viral invasion or proliferation. N=minimum of 3 independent experiments.


